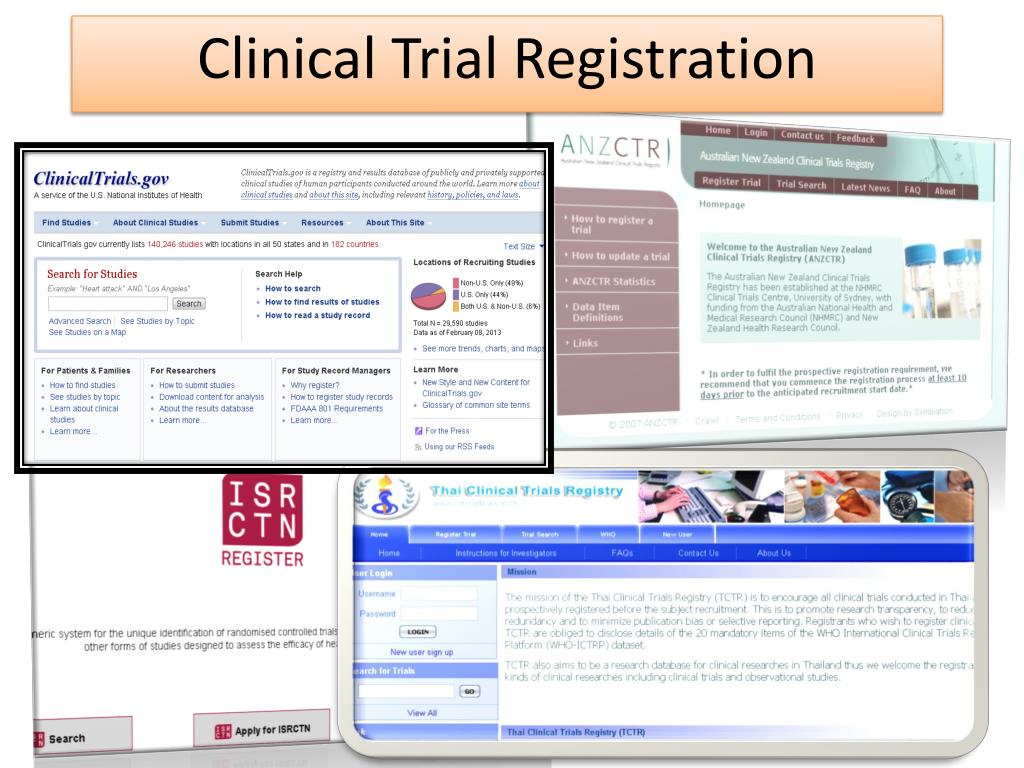
Clinical Study Online Registration - Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. When is registration and reporting required? To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. For additional information on the registration. You can access the registration site directly at:
You can access the registration site directly at: When is registration and reporting required? To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. For additional information on the registration. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and.
You can access the registration site directly at: Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. When is registration and reporting required? Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. For additional information on the registration.
How to register a research trial in Clinical Trials Registry of India
Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. For additional information on the registration. You can access the registration site directly at: When is registration and reporting required?
PPT Clinical Trial Registration PowerPoint Presentation, free
When is registration and reporting required? For additional information on the registration. To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. Clinicaltrials.gov registration is required for all federally sponsored.
How to register your clinical trials and studies at the ISRCTN registry
You can access the registration site directly at: A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. Clinicaltrials.gov registration is required for all federally sponsored clinical.
A Modernized ClinicalTrials.gov Website is Coming NCBI Insights
Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. When.
UK launches new system to achieve 100 clinical trial registration
Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. A structured online system, such as the clinicaltrials.gov.
Clinical Trial Online Courses JLI Blog
You can access the registration site directly at: To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. Clinicaltrials.gov registration is required for all federally sponsored clinical.
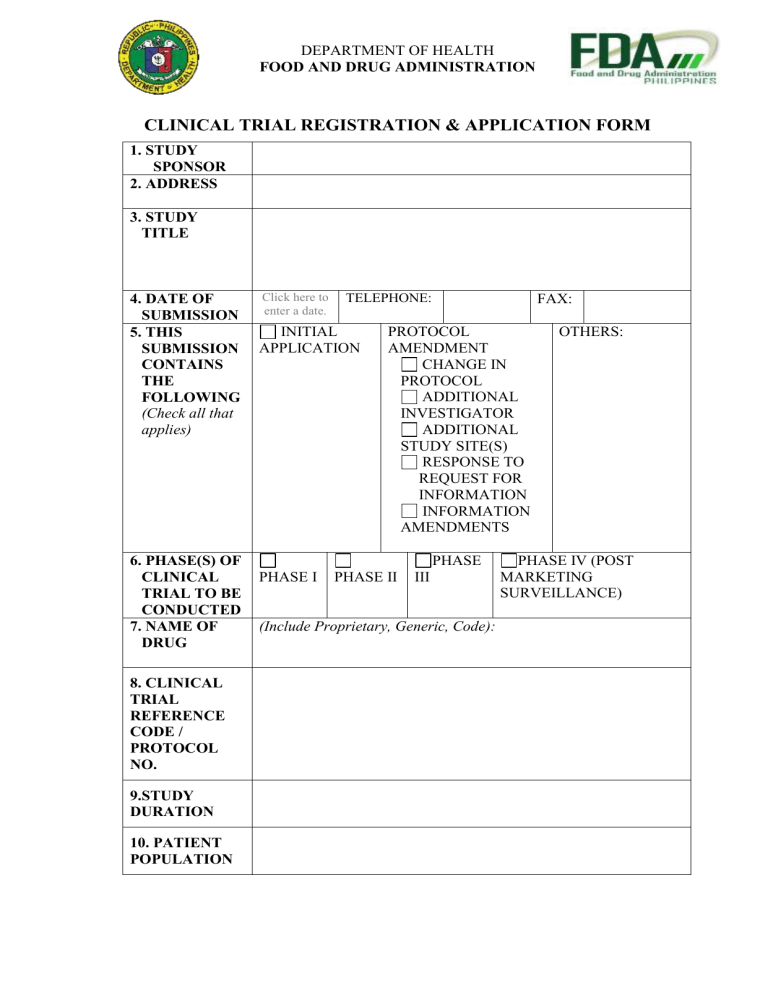
Clinical Trial Registration Application Form
Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved. When is registration and reporting.
Serbia Clinical Trial Registration Guideline Regulamedica
Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. For additional information on the registration. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. You can access the registration site directly at: Clinicaltrials.gov registration is required for all federally sponsored clinical trials or.
How to Register Clinical The Clinical Establishments Registration
Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. For additional information on the registration. You can access the registration site directly at: When is registration and reporting required? Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies.
Fillable Online clinicaldepartments musc Registration Form Clinical
A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. When is registration and reporting required? Clinicaltrials.gov registration is required for all federally sponsored clinical trials or studies. Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and. To register a trial, submit the details.
Clinicaltrials.gov Registration Is Required For All Federally Sponsored Clinical Trials Or Studies.
You can access the registration site directly at: For additional information on the registration. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and. Als.gov registration guide overview clinicaltrials.gov is a publicly available registry and results database of federally and.
When Is Registration And Reporting Required?
To register a trial, submit the details directly to any one of the primary registries in the who registry network or an icmje approved.